Clinical Transfusion Practice
BloodSafe eLearning Australia seeks endorsement and allocation of CPD points for courses from medical, nursing and midwifery colleges, societies and healthcare organisations. Further information on professional organisations and CPD points is provided below.
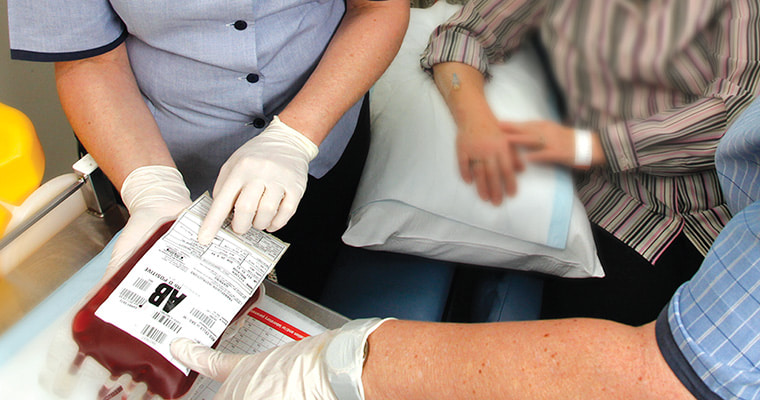
Description
This course will provide you with knowledge of safe transfusion practice and appropriate use of blood components that you can apply to your clinical setting.
Major topics covered include:
- the decision to transfuse - risks, benefits and informed consent
- pretransfusion samples – importance of patient identification and specimen labelling
- picking up blood – transporting and storage of blood
- administering blood – clinical administration and monitoring of patients
- transfusion reactions – recognising and responding to acute adverse events.
It is relevant to medical staff who prescribe transfusions, nurses and midwives who administer the transfusion and laboratory workers who want to gain knowledge of the clinical aspects of blood transfusion.
This course is based on current clinical guidelines, evidence-based material and/or expert consensus opinion, and has been developed in collaboration with leading transfusion medicine experts in Australia. It is regularly updated as changes to guidelines or accepted practice occurs.
Estimated time to complete: two hours
Course reviewed/updated: September 2018
BloodSafe eLearning Australia receives joint national funding provided by the National Blood Authority, on behalf of all Australian Governments, to enable free access by all users.
Quels sont les préréquis?
Bachelor required
Internship
Contenu du cours
Évaluation
- Répondre à chaque question. Vous avez droit à 2 tentatives pour chaque question
- Obtenir une note globale d'au moins 75%
- Terminer toutes les évaluations du cours au bout de 30 jours. Si ce n'est pas le cas, vous devrez alors répéter toutes les évaluations.
Un certificat d'achèvement sera automatiquement envoyé par e-mail à votre adresse e-mail enregistrée une fois que vous aurez terminé avec succès toutes les évaluations. Vous pouvez également voir un résumé de tous vos cours dans votre relevé de notes.
Références
Australian & New Zealand Society of Blood Transfusion Ltd. Considerations For Pre-Transfusion Immunohaematology Testing in Patients Receiving Anti CD-38 Monoclonal Antibody Therapy. 2018
Australian & New Zealand Society of Blood Transfusion Ltd. Guidelines for the implementation and use of electronic medical records for transfusion. Sydney NSW: Australian & New Zealand Society of Blood Transfusion Ltd; 1st ed. Jul 2021.
Australian & New Zealand Society of Blood Transfusion Ltd. Guidelines for prevention of transfusion-associated graft versus host disease. Sydney NSW: Australian & New Zealand Society of Blood Transfusion Ltd; Jan 2011.
Australian and New Zealand Society of Blood Transfusion. Guidelines for Transfusion and Immunohaematology Laboratory Practice. Sydney NSW: Australian & New Zealand Society of Blood Transfusion Ltd; 1st ed. Revised Jan 2020.
Australian and New Zealand Society of Blood Transfusion and Royal College of Nursing Australia. Guidelines for the Administration of Blood Products. 3rd ed. Revised Oct 2019.
Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards. Sydney: ACSQHC; 2012.
Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards, Blood Management Standard, Prescribing and clinical use of blood and blood products, Action 7.4. [cited 5 Oct 2021]. Available from: www.safetyandquality.gov.au/standards/nsqhs-standards/blood-management-standard/prescribing-and-clinical-use-blood-and-blood-products/action-704
Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards, Blood Management Standard, Prescribing and clinical use of blood and blood products, Action 7.5 [cited 05 Oct 2021]. Available from: www.safetyandquality.gov.au/standards/nsqhs-standards/blood-management-standard/prescribing-and-clinical-use-blood-and-blood-products/action-705
Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards, Communicating for Safety Standard, Correct identification and procedure matching, Action 6.5 [cited 05 Oct 2021]. Available from: www.safetyandquality.gov.au/standards/nsqhs-standards/communicating-safety-standard/correct-identification-and-procedure-matching/action-605
Australian Commission on Safety and Quality in Health Care. What is Patient Blood Management? Sydney NSW: Australian Commission on Safety and Quality in Health Care; 2016 [cited 2016 Sep 2].
The Australian Health Ministers’ Conference Statement on National Stewardship Expectations for the Supply of Blood and Blood Products. 12 November 2010.
Australian Red Cross Lifeblood. Blood Component Information Circular of Information – An extension of blood components labels. Australia: Australian Red Cross Lifeblood; March 2015.
Australian Red Cross Lifeblood. Blood conservation overview [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 October 10] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/patient-blood-management/blood-conservation
Australian Red Cross Lifeblood. Classification & incidence of adverse events [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sep 2]. Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/classification-incidence
Australian Red Cross Lifeblood. Component compatibility [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2018 [cited 2018 Jul 13]. Available from: www.lifeblood.com.au/health-professionals/products/component-compatibility
Australian Red Cross Lifeblood. What is preoperative autologous donation? [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2013. [cited 2016 Sept 2] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/patient-blood-management/blood-conservation/requirements-autologous-blood
Australian Red Cross Lifeblood. Relative risk of transfusion [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sept 2] Available from: www.lifeblood.com.au/sites/default/files/resource-library/2021-10/Relative_risk_of_transfusion.pdf
Australian Red Cross Lifeblood. Residual risk estimates for transfusion-transmissible infections [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sep 2]. Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/other-transfusion-transmitted-infections/transfusion-transmissable-infections
Australian Red Cross Lifeblood. Steps for managing suspected transfusion reactions [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sep 9] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/management-of-suspected-reactions
Australian Red Cross Lifeblood. Transfusion-associated graft-versus-host disease (TA-GVHD) [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sept 2] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/TA-GVHD
Australian Red Cross Lifeblood. Transfusion-related immune modulation (TRIM) [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sept 2] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/TRIM
Australian Red Cross Lifeblood. Transfusion-transmissible infections: variant Creutzfeldt-Jakob Disease (vCJD) [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2016 [cited 2016 Sept 2] Available from: www.lifeblood.com.au/health-professionals/clinical-practice/adverse-events/other-transfusion-transmitted-infections/vcjd
Australian Red Cross Lifeblood. Washed red blood cells negative blood [Internet]. Melbourne VIC: Australian Red Cross Lifeblood; 2013 [cited 2013 Jan 14] Available from: www.lifeblood.com.au/health-professionals/products/blood-components/red-cells
Australian Red Cross Lifeblood. Blood Book, Australian Blood Administration Handbook. 1st ed. Mar 2020.
Bolton-Maggs P, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013 Nov;163(3):303–14.
Calman K. The Health of the Nation. Brit J Hosp Med. 1996;56(4):125–6.
Coeliac Australia. Coeliac disease [Internet]. Waitara NSW: Coeliac Australia; 2016 [cited 2016 Sept 2] Available from: www.coeliac.org.au/s/coeliac-disease
Dorsey K, Moritz E, Steele W, Eder A, Stramer S. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion. 2013 Jun;53(6):1250–6.
Dzik W. Technology for Enhanced Transfusion Safety. Hematology Am Soc Hematol Educ Program. 2005:476–82.
Farmer S, Towler S, Leahy M, Hofmann A. Drivers for change: Western Australia Patient Blood Management Program (WA PBMP), World Health Assembly (WHA) and Advisory Committee on Blood Safety and Availability (ACBSA). Best Pract Res Clin Anaesthesiol. 2013 Mar;27(1):43–58.
The Kirby Institute, UNSW Sydney, and Australian Red Cross Blood Service. Transfusion-transmissible infections in Australia: 2016 Surveillance Report.
Liumbruno G, Bennardello F, Lattanzio A, Piccoli P, Rossetti G. Recommendations for the transfusion of red cells. Blood Transfus. 2009 Jan;7(1):49–64.
National Blood Authority Australia. Ensuring Supply. Canberra ACT: National Blood Authority Australia; 2016 [cited 2016 Sep 2]. Available from: www.blood.gov.au/ensuring-supply
National Blood Authority Australia. Patient Blood Management Guidelines. Canberra ACT: National Blood Authority Australia; 2016 [cited 2016 Sep 2]. Available from: www.blood.gov.au/pbm-guidelines
National Blood Authority Australia. Patient Blood Management Guidelines: Module 2 – Perioperative. Commonwealth of Australia, Canberra, 2011.
National Blood Authority Australia. Patient Blood Management Guidelines: Module 3 – Medical. Commonwealth of Australia, Canberra, 2012.
National Blood Authority Australia. Patient Blood Management Guidelines: Module 4 – Critical Care. Commonwealth of Australia, Canberra, 2013.
National Blood Authority Australia. Patient Blood Management Guidelines: Module 6 – Neonatal and Paediatrics. Commonwealth of Australia, Canberra, 2016.
Pasricha S, Flecknoe-Brown S, Allen K, Gibson P, McMahon L, Olynyk J, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Med J Aust. 2010; 193(09):525–32.
PHB Bolton-Maggs (Ed) D Poles et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report (2016).
Quach H, Benson S, Haysom H, Wilkes A-M, Zacher N, Cole-Sinclair M, et al. Considerations for pre-transfusion immunohaematology testing in patients receiving the anti-CD38 monoclonal antibody daratumumab for the treatment of multiple myeloma. Internal medicine journal. 2018;48(2):210-20.
Subramaniyan R, Satheshkumar R, Pereira KR. Role of daratumumab in transfusion medicine: a must know entity. Revista Brasileira de Hematologia e Hemoterapia. 2017;39(4):375-8.
Transfusion Science Standing Committee, Australian & New Zealand Society of Blood Transfusion Ltd. Guidelines for transfusion and immunohaematology laboratory practice. Sydney NSW: Australian & New Zealand Society of Blood Transfusion Ltd; 1st ed. Revised Jan 2020.
Équipe de développement
Approbation du contenu du cours
D'autres cours de la même catégorie
Voici certains cours qui pourront vous intéresser


